- Therma Bright’s AcuVid COVID-19 test detects SARS-CoV-2 variants of concern; Brazilian P.1 and P.2 and UK B.1.1.7
- Results of a preliminary test showed 100% sensitivity when tested on previously sequenced variant samples
- Therma Bright also announces that it has received TSXV approval for debt settlements previously announced on January 15, 2021
- The company has engaged a consultant to set up a network of manufacturing facilities for the worldwide distribution of its current and future COVID-19 products
- Therma Bright has engaged a consultant to provide corporate communications and branding services
- Therma Bright is focused on providing consumers and medical professionals with quality medical devices
- Therma Bright (THRM) opened trading at C$0.49 per share
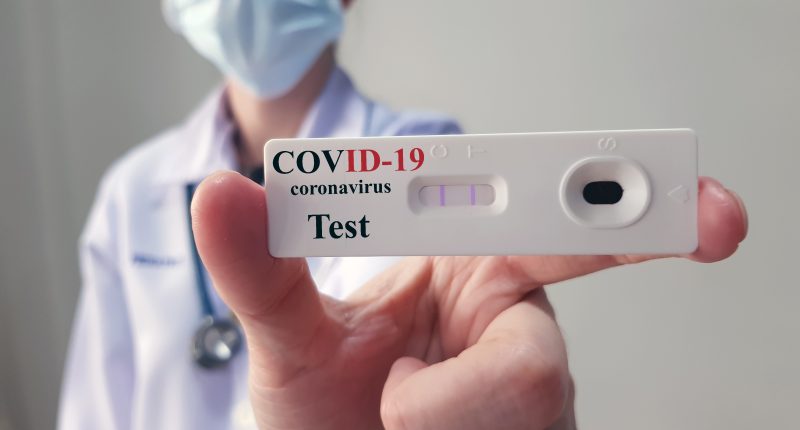
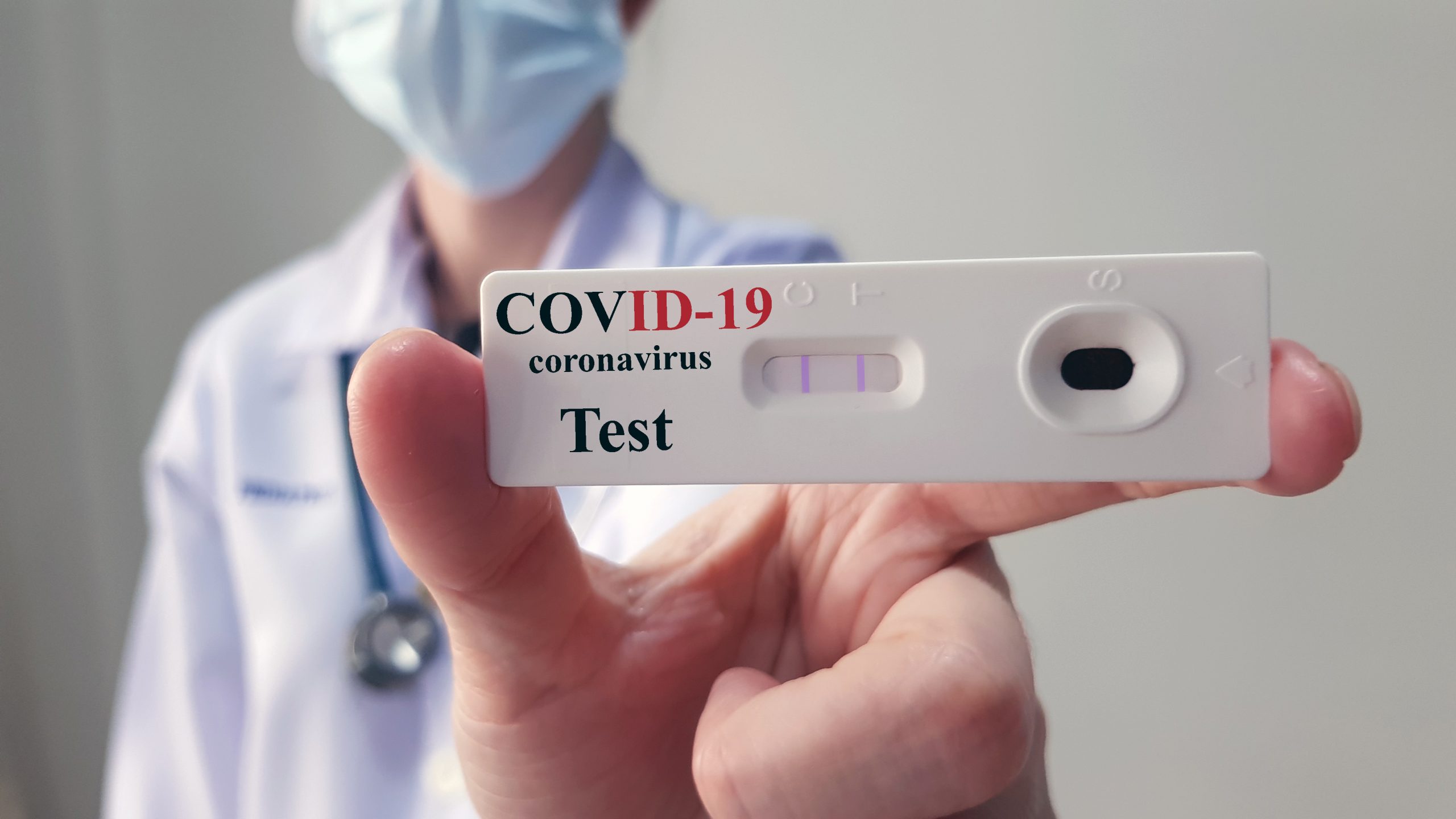
Therma Bright (THRM) is pleased to announce successful preliminary results with its AcuVid™ COVID-19 Rapid Antigen Saliva Test to detect the SARS-CoV-2 Variants of Concern (“VOC”).
The variants detected include the Brazilian P.1 and P.2 and UK B.1.1.7.
The results were obtained during the performance study currently being conducted in Brazil at the Federal University of Minas Gerais (UFMG).
The P.1 SARS-CoV-2 variant was first seen in Manaus, the capital of the state of Amazonas in Brazil. Initial research suggested the virus arose late last year and began spreading in November. It quickly became the dominant strain, leading many in the country to believe it could infect people who had already been infected with the initial strain earlier in the year.
After initially containing COVID-19, many European, Asian and other countries, including Canada, are experiencing a resurgence of COVID-19 in large part due to the evolving SARS-CoV-2 Variants of Concern which are either easier to transmit, cause more severe disease, or both.
Therma Bright successfully completed a preliminary study of the AcuVid COVID-19 Rapid Antigen Saliva Test to detect the Brazilian P.1 and P.2 variants and the UK B1.1.7 variant. The results showed 100% sensitivity when tested on previously sequenced variant samples.
Over the coming weeks, additional studies will be conducted on a larger number of subjects in Brazil under the leadership of Dr. Ricardo Fujiwara in real-life hospital settings to confirm the initial results.
Mr. Rob Fia, CEO of Therma Bright commented,
“We are pleased that our AcuVid™ test detects the most important circulating Variants of Concern that are responsible for increased infections and death rates. We feel our test will be able to detect any new variants as they arise. Being able to detect these is crucial, as the number of people being infected with VOCs is rapidly rising worldwide.”
Therma Bright also announces that it has received TSXV approval for debt settlements previously announced on January 15, 2021.
The company has issued an aggregate of 866,664 common shares which are subject to a hold period expiring August 14, 2021.
Therma Bright has negotiated a debt settlement with an arm’s length creditor. Subject to acceptance by the TSXV, the company has agreed to settle outstanding debt of $100,000 by issuing 200,000 units at a price of $0.50 per unit. Each unit is comprised of one common share and one-half of one common share purchase warrant. Each whole warrant will entitle the creditor to purchase one common share for two years from the date of issue at a price of $0.60. All securities issued in relation to this debt settlement are subject to a hold period expiring four months + one day after the date the securities are issued.
Therma Bright also announces that it has engaged a consultant to set up a network of manufacturing facilities for US, Canada and European distribution for its current and future COVID-19 products. Therma Bright intends to issue up to 500,000 share purchase warrants to the consultant for the services provided, to be paid in tranches upon certain milestones being met.
In addition, the company has engaged a consultant to provide corporate communications and branding services. Therma Bright intends to issue up to 750,000 share purchase warrants to the consultant for the services provided, to be paid in tranches upon certain milestones being met. The warrants listed above will be exercisable for two years and the exercise price will be determined at the time of each issue based on the volume-weighted average closing price of the Company’s common shares on the TSXV for the 5 trading days immediately preceding the date of issue of the warrants.
The company is not making any express or implied claims that its test product can eliminate or cure COVID-19 or the SARS-CoV-2 virus.
Therma Bright is focused on providing consumers and medical professionals with quality medical devices that address their unmet needs.
Therma Bright (THRM) opened trading at C$0.49 per share.