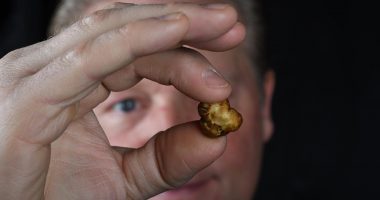
- Nova Mentis Life Science Corp. (NOVA) is strengthening its IP portfolio with the filing of provisional patent applications to protect data from its psilocybin preclinical studies
- It also seeks protection for the company’s therapeutic paradigm for treating neuroinflammatory disorders with psilocybin and psilocybin-based tryptamine derivatives
- The provisional patent applications filed with the U.S. Patent and Trademark Office relates to diagnosis, monitoring and treating neuroinflammatory diseases and conditions with psychoactive tryptamine derivatives
- Nova Mentis Life Science Corp. is a Canadian-based biotechnology company
- Nova Mentis Life Sciences Corp. was unchanged, trading at $0.075 at 11:45 AM ET
Nova Mentis Life Science Corp. (NOVA) has filed provisional patent applications to protect data it obtained during psilocybin preclinical studies.
The company also seeks protection for its therapeutic paradigm for treating neuroinflammatory disorders with psilocybin and psilocybin-based tryptamine derivatives.
“The filing of these patent applications represents a major milestone in NOVA’s psilocybin research program,” noted Dr. Marvin S. Hausman, MD, Chairman of NOVA’s Scientific Advisory Board.
“The company is now in a position to reveal the breakthrough preclinical psilocybin results uncovered in its treatment of fragile X syndrome (FXS), and the data is scheduled to be published in a peer reviewed journal and presented at the upcoming 18th NFXF International Fragile X Conference in July 2022,” he added.
The provisional patent applications filed with the U.S. Patent and Trademark Office related to diagnosing, monitoring and treating neuroinflammatory diseases and conditions with psychoactive tryptamine derivatives.
NOVA’s psilocybin formulation was evaluated in two distinct rat models of autism in the laboratory of Dr. Viviana Trezza, Rome, Italy, and demonstrated psilocybin proof of efficacy and safety.
In February 2022, the company announced it had completed a preclinical study that confirmed oral microdose psilocybin as a potential treatment option for ASD and FXS.
The research found that a repeated low dose of NOVA’s proprietary psilocybin drug (NM-1001) significantly modulated behavioural and cognitive defects, such as recognition memory, in a genetic model of FXS.
The ability to penetrate the unique genetic language underlying the development of chronic diseases and assess therapeutic responses will assist NOVA in obtaining psychedelic drug approval from the regulatory agencies.
Nova Mentis Life Science Corp. is a Canadian-based biotechnology company and global leader in developing diagnostics and psilocybin-based therapeutics for neuroinflammatory disorders.
Nova Mentis Life Sciences Corp. was unchanged, trading at $0.075 at 11:45 AM ET.