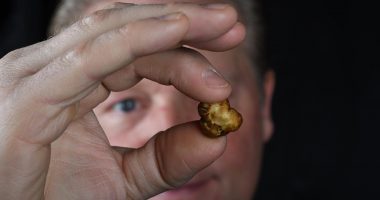
- Nova Mentis (NOVA) has completed the production of psilocybin microdose capsules in partnership with the Toronto Institute of Pharmaceutical Technology
- TIPT and NOVA have recently completed the manufacturing of an engineering quantity of the psilocybin microdose capsules that were used to confirm product specifications
- NOVA is currently preparing to submit a clinical trial application to Health Canada for a Phase 2A study evaluating psilocybin microdose therapy for fragile X syndrome
- The company has formed a tactical partnership with KGK Science Inc. to develop its psilocybin drug portfolio in Canada
- Nova Mentis Life Science Corp. is a Canadian-based biotechnology company
- Nova Mentis Life Science Corp (NOVA) is unchanged on the day, trading at C$0.035 per share at 2:30 pm ET
Nova Mentis (NOVA) has completed the production of psilocybin microdose capsules in partnership with the Toronto Institute of Pharmaceutical Technology (TIPT).
TIPT and NOVA have recently completed the manufacturing of an engineering quantity of the psilocybin microdose capsules that were used to confirm product specifications for the capsule. Production of the first stability lot followed, and capsules are currently undergoing release testing this week to generate data required for a clinical trial application.
NOVA is currently preparing to submit a clinical trial application to Health Canada for a Phase 2A study evaluating psilocybin microdose therapy for fragile X syndrome (FXS), the leading genetic cause of autism spectrum disorder (ASD). NOVA’s recent search results showed that a very low microdose formulation of the company’s psilocybin drug (NM-1001) modulated behavioural and cognitive defects in a genetic model of FXS.
“A major success for NOVA to achieve this critical drug development accomplishment! Our team is incredibly dedicated and focused on achieving our planned milestones as we work towards meeting our mission of diagnosing and treating chronic conditions such as autism spectrum disorder and fragile X syndrome,” said Jacqueline McConnell, NOVA’s Chief Operating Officer.
The company has formed a tactical partnership with KGK Science Inc. to develop its psilocybin drug portfolio in Canada. KGK is a leading North American contract research organization based in London, Ontario, that provides high-quality clinical research trials with a focus on the nutraceutical, cannabis and emerging psychedelic industries.
Both companies plan to jointly submit the Health Canada clinical trial application.
NOVA is the first biotech company to achieve orphan drug designation in both the United States and the European Union for the use of psilocybin in the treatment of FXS. NOVA has manufactured a large supply of >98 per cent pure psilocybin for clinical studies and commercialization following drug approval.
Nova Mentis Life Science Corp. is a Canadian-based biotechnology company and leader in developing diagnostics and psilocybin-based therapeutics for neuroinflammatory disorders.
Nova Mentis Life Science Corp (NOVA) is unchanged on the day, trading at C$0.035 per share at 2:30 pm ET.