- Marvel (MRVL) has begun its 10-day, dose-ranging toxicology study for its lead molecule MB-204
- The maximum tolerated single-dose studies have been completed. FDA guidelines for drug development next prescribe a one-week multiple-dosing study required to run the industry standard four-week good lab practice toxicology studies
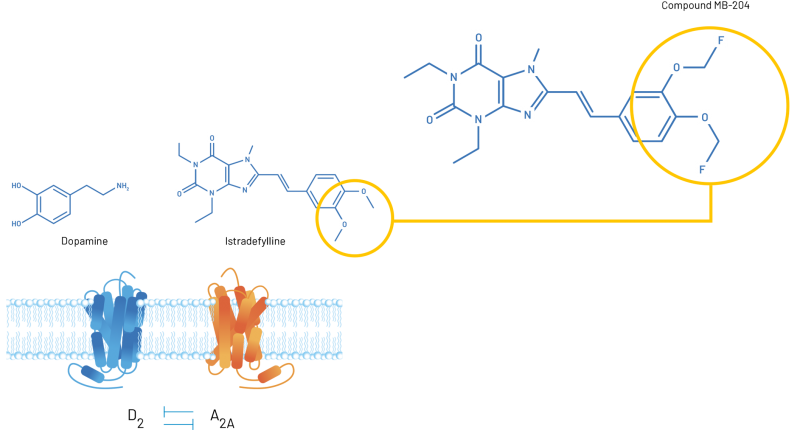
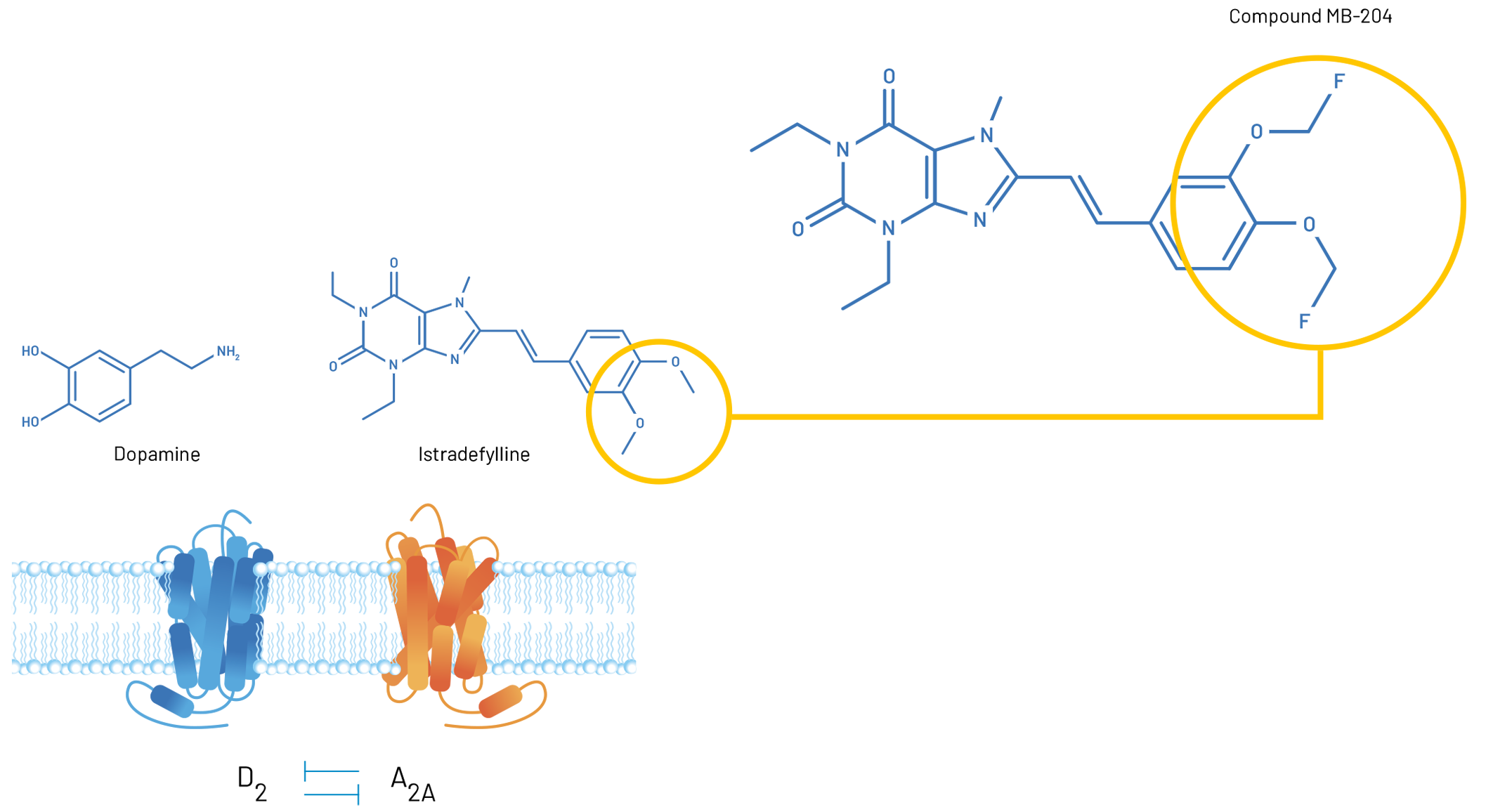
- MB-204 is a fluorinated derivative of the U.S. FDA-approved adenosine A2a receptor antagonist, Istradefylline, a highly active derivative of caffeine, which has been associated with a reduced risk of developing Parkinson’s disease, and Alzheimer’s disease and improved mood and concentration
- Marvel Biosciences (MRVL) is unchanged, trading at $0.14 per share as of 9:55 am ET
Marvel (MRVL) has begun its 10-day, dose-ranging toxicology study for its lead molecule MB-204.
MB-204 has shown positive and promising data addressing depression and anxiety, as well as its potential benefit in protecting patients’ vital organs while going through chemotherapy treatments. As the maximum tolerated single-dose studies have been completed, FDA guidelines for drug development next prescribe a one-week multiple-dosing study required to run the industry standard four-week good lab practice toxicology studies. The studies are all required by the FDA prior to entering FDA Phase I human trials.
MB-204 is a fluorinated derivative of the U.S. FDA-approved adenosine A2a receptor antagonist, Istradefylline. Both Istradefylline and MB-204 are highly active derivatives of caffeine, which has been associated with a reduced risk of developing Parkinson’s disease, and Alzheimer’s disease and improved mood and concentration.
Rod Matheson, CEO of Marvel Biosciences, explained that the team continues to achieve its milestones on schedule with progress in getting the company’s key asset, MB-204, ready to enter human trials in early 2023.
“MB-204 has the potential to target a very significant market. We will continue to closely monitor the effects of this lead asset drug and will seek all opportunities to maximize shareholder value from our key asset.”
Dr. Mark Williams, Chief Science Officer of Marvel Biosciences, added that this is the final milestone the company needs to run to identify the best doses in the upcoming four-week good lab practice study, which in combination with earlier animal studies, will enable the team to commence human clinical testing of MB-204.
“We are close to completing the seven-day dose-ranging study using rats as well and will update our stakeholders on our progress shortly.”
Marvel Biosciences is a pre-clinical stage pharmaceutical development biotechnology company that utilizes synthetic chemical derivatives of known, off-patent drugs to develop new drugs.
Marvel Biosciences (MRVL) is unchanged, trading at $0.14 per share as of 9:55 am ET.