- First patient has enrolled in ARCHER – the company’s Phase II study to examine the safety and tolerability of CardiolRx
- ARCHER has received regulatory clearance in multiple jurisdictions, including IND authorization from the U.S. FDA
- The study is expected to enroll 100 patients at major cardiac centers in North America, Europe, Latin America, and Israel
- Cardiol Therapeutics Inc. (CRDL) is up 0.56 per cent to C$1.81 per share at 11:00 am EDT
Cardiol Therapeutics (CRDL) has announced that the first patient has been enrolled in ARCHER – the company’s Phase II trial.
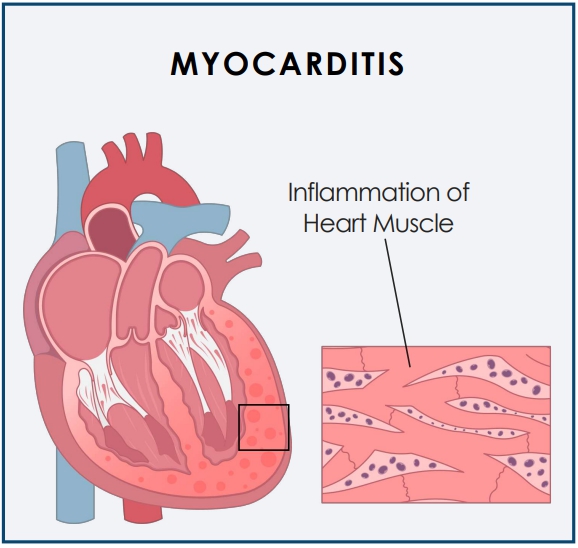
The trial is designed to study the safety and tolerability of CardiolRx and its impact on myocardial recovery in patients presenting with acute myocarditis.
CardiolRx is a pharmaceutically produced oral cannabidiol formulation being developed for the treatment of acute and chronic inflammation associated with heart disease.
Dennis McNamara, MD, MS, Professor of Medicine at the University of Pittsburgh, Director of the Center for Heart Failure Research at the University of Pittsburgh Medical Center, and Chair of the study steering committee commented,
“We have long suspected that it is the response to injury that needs to be addressed to improve outcomes in myocarditis. Given its impact limiting these inflammatory mechanisms, we believe cannabidiol has the potential to truly benefit patients with this condition. I am pleased this important milestone has now been achieved and the ARCHER study, designed to investigate CardiolRx’s therapeutic potential in myocarditis, is formally underway.”
ARCHER has received regulatory clearance in multiple jurisdictions, including Investigational New Drug Application (IND) authorization from the U.S. Food and Drug Administration, and is expected to enroll 100 patients at major cardiac centers in North America, Europe, Latin America, and Israel.
Myocarditis is an acute inflammatory condition of the heart muscle (myocardium) characterized by chest pain, impaired cardiac function, atrial and ventricular arrhythmias, and conduction disturbances.
Cardiol Therapeutics is a clinical-stage life sciences company focused on the research and clinical development of cannabidiol as an anti-inflammatory and anti-fibrotic therapy for the treatment of cardiovascular diseases.
Cardiol Therapeutics Inc. (CRDL) is up 0.56 per cent to C$1.81 per share at 11:00 am EDT.