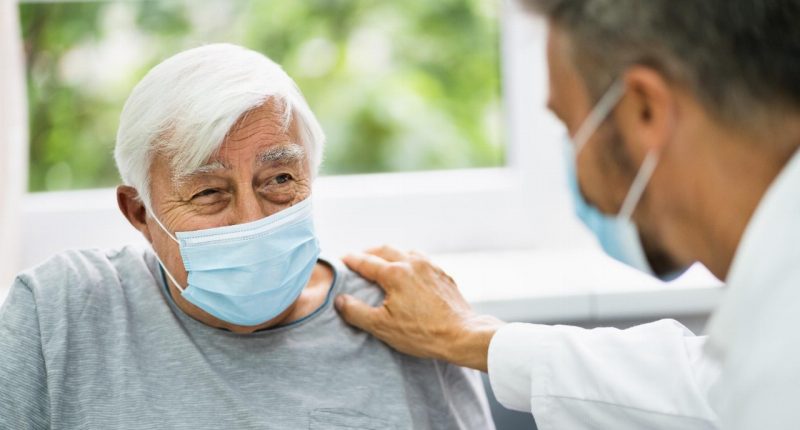
- Algernon Pharmaceuticals (AGN) has announced topline data from its Phase 2b/3 COVID-19 trial of Ifenprodil
- This phase sought to identify approvable U.S. FDA endpoints that merit moving forward into a Phase 3 study
- The 20 mg dose Ifenprodil treatment arm experienced no mortality compared to 3.3 per cent in the untreated control arm
- All patients with low blood oxygen levels in the 20 mg treatment arm returned to normal on day 4 compared to day 9 for the untreated arm
- Patients in the 20 mg dose arm also spent less time in the ICU compared to patients in the untreated arm
- Algernon is a drug re-purposing company that investigates approved drugs for new disease applications
- Algernon Pharmaceuticals (AGN) is down 15.25 per cent and is currently trading at C$0.25 per share
Algernon Pharmaceuticals (AGN) has announced topline data from its Phase 2b/3 COVID-19 trial of Ifenprodil.
Ifenprodil is an orally delivered small molecule originally developed by Sanofi to treat peripheral circulatory disorders.
The purpose of this phase was to identify approvable U.S. FDA endpoints that merit moving forward into a Phase 3 study.
At day 15 of the study, the last day of treatment, there was zero per cent mortality in the 20 mg dose Ifenprodil treatment arm compared to 3.3 per cent in the untreated control arm. Approximately 1900 patients would need to be enrolled for a Phase 3 trial to confirm this result.
All patients with low blood oxygen levels in the 20 mg treatment arm returned to normal on day 4 compared to day 9 for the untreated arm. Approximately 450 patients would be required to confirm this result in a Phase 3 trial.
Patients in the 20 mg treatment arm also spent less time in the ICU compared to patients in the untreated arm. However, Algernon cautions that it detected additional variables and needs to confirm these numbers with additional analysis.
The WHO score, the primary default endpoint for the study, showed a similar mean in all patients in all study arms.
The company observed no significant changes in other secondary endpoints, namely the time to hospital discharge, rates and duration of mechanical ventilation, or the National Early Warning Score.
Algernon investigated a 20 mg and 40 mg dose of Ifenprodil. Based on the initial data review, no significant changes were observed in the 40 mg dose group.
The company will discuss these results with the U.S. FDA and intends to publish the complete data set in a peer-reviewed journal at a later time.
Christopher J. Moreau, Algernon’s CEO, commented,
“The company has done a tremendous amount of work in a very short period of time to get to this stage to see if Ifenprodil could help in the world’s fight against COVID-19. We look forward to completing our data review and receiving feedback from the U.S. FDA.”
The company is not making any claims that Ifenprodil has the ability to eliminate, cure or contain COVID-19 or the SARS-2 Coronavirus at this time.
Algernon is a drug re-purposing company that investigates already approved drugs for new disease applications.
Algernon Pharmaceuticals (AGN) is down 15.25 per cent and is currently trading at C$0.25 per share as of 9:30 am ET.